Groundbreaking Personal Protective Equipment
Our Personal Protective Equipment, ProtectivAir®, was conceived to protect the world population from infectious airborne pathogens. Influenza remains the most dangerous respiratory disease according to the WHO, but the emergence of the Covid19 pandemic has naturally focussed attention on the SARS-CoV-2 virus.
Our partnership with Mackwell Health, another UK company will enable us to complete development in the shortest possible timescale, so that Health Workers, First Responders and other critical groups, can be better protected against the current pandemic, seasonal influenza and any other emerging respiratory disease.
What is ProtectivAir®
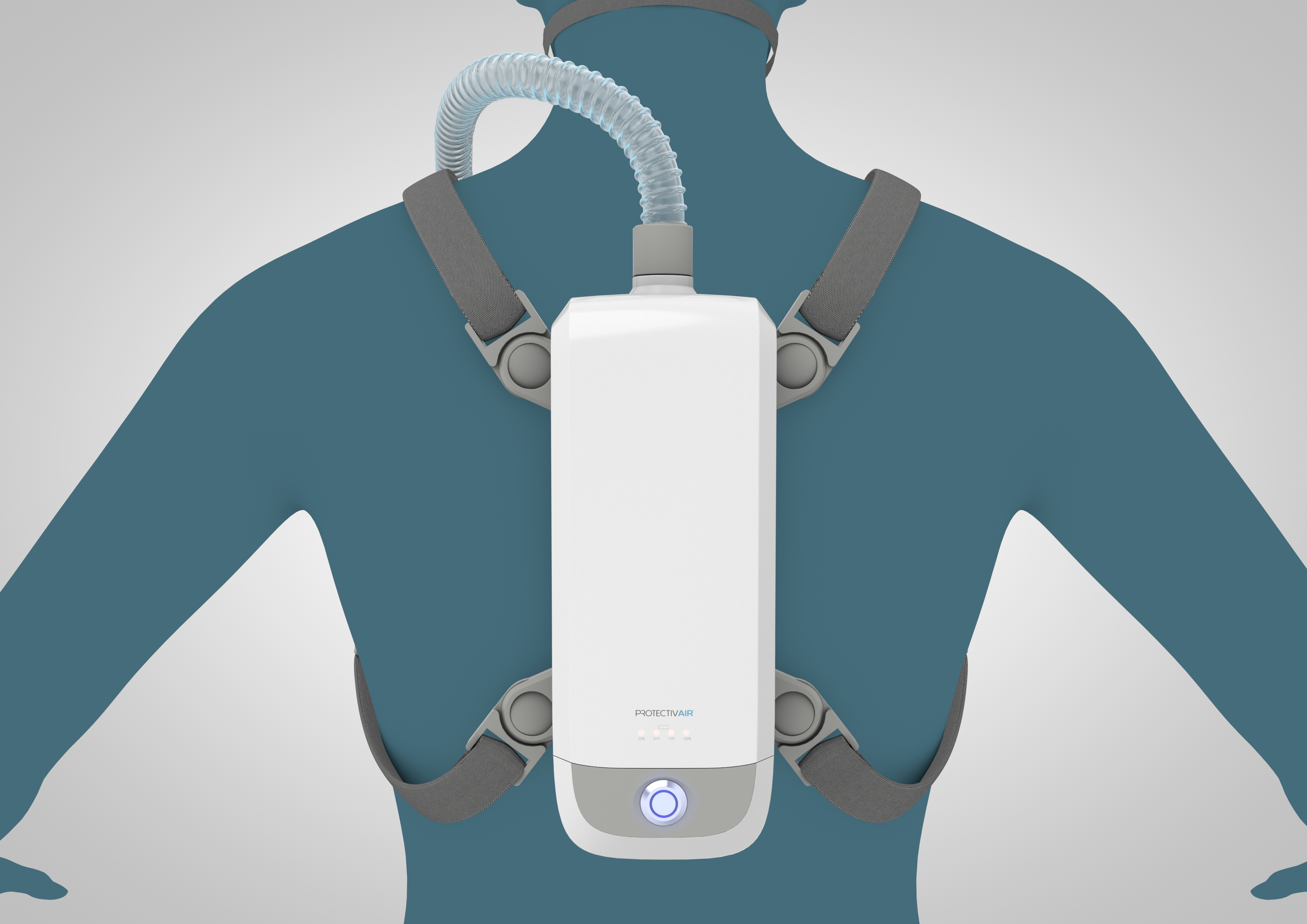
ProtectivAir® is a wearable, filter-free, breathing device that biologically sterilizes inhaled air, providing immediate protection against any airborne pathogen, even mutations.
If a pathogen has not yet been identified, or is a mutation of a known pathogen, ProtectivAir® will still protect. This allows time for the identification and subsequent production of a vaccine.
This technology has been confirmed in tests at Public Health England,
Porton Down. To view report click here
ProtectivAir® Patented Technology
Within the filter free irradiation chamber, energetic UVc photons pass through the infecting micro-organisms, damaging their nucleic acid (RNA or DNA) which disables their ability to replicate and infect the body.
The use of UVc in disinfection/decontamination is widely accepted and all published data confirms that UVc is an extremely effective germicidal agent.
Current Description – ProtectivAir®
| Dimensions: | 235mm x 92mm x 90mm |
| Power source: | Battery operated |
| Battery life: | 6 hours, re-chargeable and hot swappable, with low charge warning indicators. |
| Usage: | Re-useable device, 5+ year with maintenance |
| Cleaning: | Internal self-sterilizing (external antibacterial wipe) |
| Portability: | ProtectivAir® can be worn on a belt or harness |
| Users: | Suitable for adults and children |
| Wearer Profile: | First Responders, Health and Social Care Workers, Dentists and dental Hygienists, Aircraft workers and passengers, Key Public and Government services, Food and Pharmaceutical Supply, Transport, Key Utilities, Key Communication and Financial Services |
| Accessories: | A comfortable, interchangeable, nose and mouth mask supplied with a flexible hose that attaches to the device. |
| Protection Level: | Using an example from viable Influenza A tests conducted at PHE, a 10-log reduction in viral particles is achieved at normal breathing rates i.e. less than 1 in 10,000,000,000 particles remains viable |
| Training: | Minimal user training required |
Wearer Suitability Study
One of the targeted end users of The Medi-Immune ProtectivAir® are healthcare
professionals who work in an environment potentially exposed to airborne pathogens
e.g. first responders, emergency services, military, and public servants. It is also
envisaged that the general public will benefit from its use, particularly those with
compromised immune systems.
The conducted usability study was structured around simulated tasks, based on
working scenario for 4 volunteers, all of whom are research nurses with varying levels
of experience.
All the participants were able to carry out the tasks proficiently whilst wearing the
ProtectivAir® device.
Overall there was a positive response from the participants to the PPE device tested. It
was described as “user-friendly” and “more comfortable than other current
masks”
The participants appreciated the purpose of the ProtectivAir® suggesting that if staff
were aware of the benefits, it would likely be welcomed, especially as it is more
comfortable than other masks.
The participants were able to complete all the tasks required of them, whilst wearing
the PPE device. Therefore, there is a completion success rate of 100% for the
representative end users.